The job of a data scientist is to look at data and find patterns to explain what is going on. The Covid crisis has been a textbook example of "patterns" at odd with the narrative as we documented extensively on this blog.
In the 1960s following the assassination of JFK, the CIA invented the concept of "conspiracy theory" in order to dismiss more easily the proof that some people were bringing to the table that a lone assassin could not possibly have killed a president. The Warren commission wrote an absurd report full of unbelievable facts such as bullets bouncing and reentering bodies at different angles and whoever exposed the absurdity was labelled a conspiracy theorist. No need to disprove anything for the well oiled propaganda machine.
Move forward 50 years and admire the progress of social engineering. Now exposing the lies of the Covid campaign for this is what is was, a campaign, got you the fresh label of anti-vax, similar to the label of global warming denialist for looking at actual data related to the climate.
The power of such term is strong enough that you do not need to discuss any troubling data. Whatever doesn't fit the narrative is preemptively cancelled on ideological ground. If fact, if you put it in those terms, you suddenly realize that it applies to almost every controversial issue nowadays. Either you conform or you are a heretic who deserves to be silenced.
And on and on, we move from subject to subject, crimes are exposed, whistleblowers are shunned, the narrative is restored. They "own" the science!
Except that they don't. Science is not yet dead and the truth can still be exposed for all to see. The only thing they own is the medias that fewer and fewer people listen to.
Now bird flu. The latest iteration of genetic manipulation being presented as natural evolution even though absolutely nothing is natural about the virus and its behavior as exposed below...
What should be abundantly clear by now is that the problem we have is not "climate", "virus", "terrorists" or any other emerging factor but a more fundamental one of a system gone rogue using the scare of the day to push an agenda of world domination and enslavement of bodies and minds.
The good thing is that more and more people are coming to this view and understand what is going on. The less good one is that the people doing these things are ruthless and will stop at nothing. They will play with fire, nuclear, AI or genetic engineering until eventually some sparks ignite a global conflagration. Now faced with a collapsing financial system, why not push all the buttons at once. Isn't it the modern equivalent of upturning the apple cart? What could go wrong?
Authored by Yuhong Dong, M.D., Ph.D. and Xiaoxu Sean Lin, M.D., Ph.D. via The Epoch Times,

In the past six months, bird flu has surprised scientists at least twice.
Bird flu viruses have circulated mainly in birds for a long time. However, in early December 2023, an outbreak occurred in U.S. dairy cows, even though cattle are not typically susceptible to avian influenza A, the bird flu virus.
In late March, a U.S. dairy farm worker was infected by a H5N1 virus from a cow.
On May 22, a second human case of H5N1 infection was reported with prior exposure to infected dairy cows in Michigan.
On the same day in May, an Australian child was infected by an H7 strain, another subtype of influenza A known to cause human infections.
Since bird flu infections in humans are rare, these incidents have raised significant concern among scientists.
Why is this happening, and how concerned should we be?
This article aims to avoid unnecessary fear about a potential future pandemic. Instead, we encourage people to think rationally and make appropriate adjustments for the future.
Rapid Spread in Birds
The history of the H5N1 virus family can be traced back to 1996 when it was first discovered in a sick goose in the Guangdong province of China.
H5N1 has evolved, resulting in different genetic lineages (clades) as they mutate, similar to a typical pattern of behavior for RNA viruses such as the ever-emerging COVID-19 variants. In 2013, the H5N1 clade 2.3.4.4b emerged. Since then, it has spread rapidly to nearly 100 countries across Asia, Europe, Africa, and America, becoming the most dominant clade and causing significant losses to the poultry industry.

In December 2021, this particular clade 2.3.4.4b, was first identified in wild birds in the United States.
The clade quickly mixed with other circulating influenza A viruses in wild birds in North America. This resulted in viral reassortment and recombination of genes and exhibiting diverse characteristics. Many of these variants cause severe illnesses in mammals, significantly affecting their nervous system.
The Jump to Cows
The avian influenza virus, commonly called the bird flu virus, belongs to the flu virus family. Flu viruses have many natural hosts, including ducks, geese, swans, gulls, terns, waders, pigs, and horses.
Certain types of flu viruses typically infect specific hosts and do not usually jump from one host to another.
There is a wide variety of bird flu viruses, ranging from H1 to H19, but they have mostly remained in birds and animals, rarely affecting humans.
This changed with the H5N1 clade 2.3.4.4b.
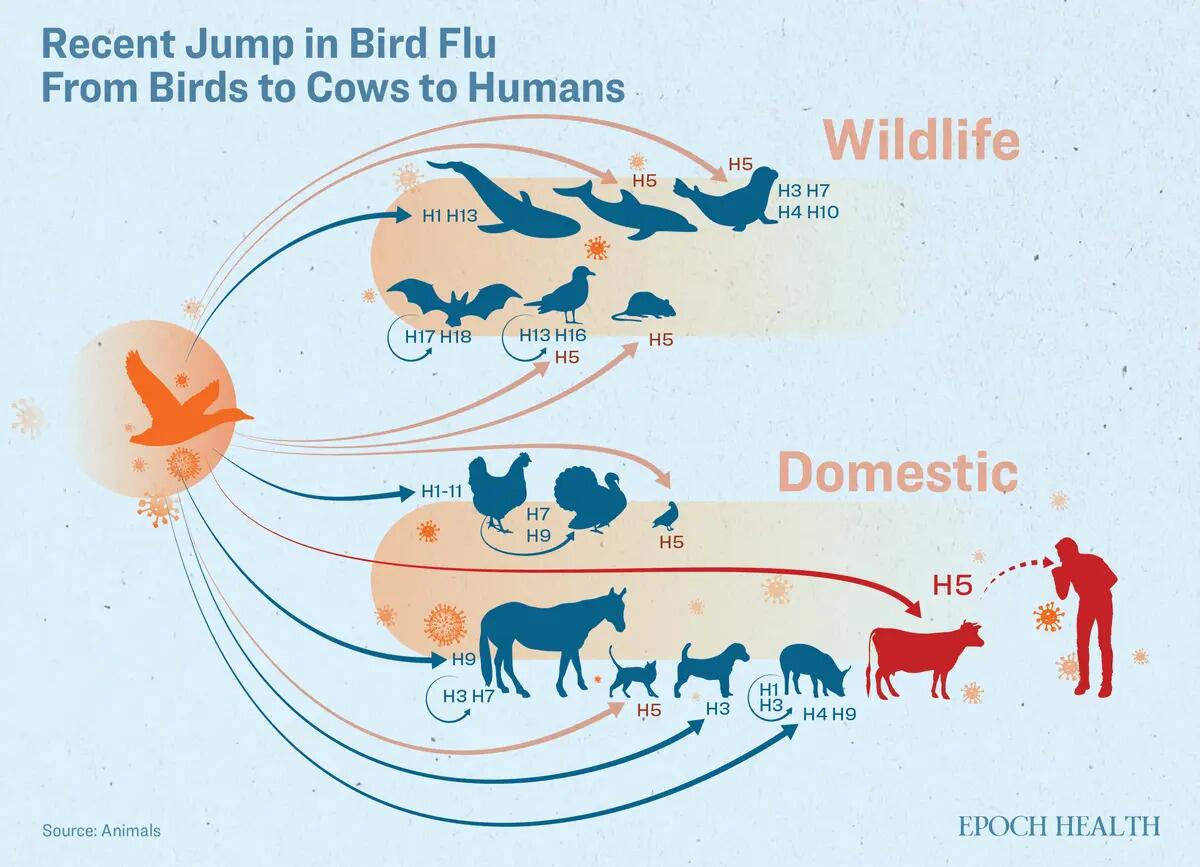
This clade became concerning because of their frequent spillover events. A spillover event occurs when a virus from one normal host reservoir jumps into a new or different host species, for example, jumping from a bird to a horse or cattle.
Since December 2023, the highly pathogenic H5N1 clade 2.3.4.4b viruses have been reported to spread in dairy cows in multiple U.S. states, according to the U.S. Department of Agriculture (USDA) and Centers for Disease Control.
From early this year, some cows have been producing less milk and eating less. It was later confirmed that the H5Nx clade 2.3.4.4b viruses were present in both the cows’ milk and nasal samples. The USDA reported an outbreak in this clade in cows for the first time.
The December USDA preprint reveals that the same viral strain was found in dairy cows that have no known connection to the infected herds. This suggests that the transmission in cows has already started quietly, and asymptomatic cows likely contributed to the rapid spread of the virus.
As of May 28, there were 67 herds infected by the H5N1 virus in nine states. Despite the low number of infected herds, this could indicate that it is no longer just a spillover event, but rather a significant expansion of host tropism. The concern is when a large-scale outbreak might occur.
Furthermore, as dairy cows often live in close proximity to humans, infections in cows may also impact human health.
The Likely Jump to Humans
Although bird flu infections in humans have been rare, they can happen.
In the past 20 years, there have been sporadic human infections with the H5N1 virus. There have been 888 infected patients, resulting in 463 deaths reported across 23 countries. The majority of cases have occurred in Egypt, Indonesia, and Vietnam. These cases have resulted in a cumulative case fatality rate of more than 50 percent, based on data collected by the World Health Organization.
Since these cases are mostly scattered throughout Asia, they haven’t received much public attention in Western countries until recently.
In April 2022, a case was confirmed in a Colorado poultry worker who has since recovered. This was the first known case of H5N1 infection transmitted from poultry to a human in the United States.
The second human case in the United States didn’t occur until late March. A dairy farm worker in Texas showed symptoms of hemorrhagic conjunctivitis in both eyes and was confirmed to be infected by the H5N1 clade 2.3.4.4b. He had no respiratory symptoms and fully recovered within a few days.
However, this person reported no contact with sick or dead birds but had close exposure to sick dairy cows. The cows showed decreased milk production, reduced appetite, fever, and dehydration, suggesting H5N1 infection.
This was the first report in the United States of the highly pathogenic avian influenza H5N1 virus suspected of transmitting from a mammalian animal species to a human.
These cases have alerted scientists, as they suggest that the virus may have acquired the ability to spread between mammals and potentially infect humans.
If a highly pathogenic H5N1 virus were to develop the ability to spread easily among humans, including through human-to-human transmission, it could have a significant impact on the human population, given the high mortality rate observed in previous cases.
Since these are the only two confirmed U.S. cases of cow-to-human transmission, the full extent of similar infections and the mortality rate remain unknown.
The spillover from one species to another typically happens naturally through the food chain. For instance, it can happen when infected birds are eaten by another species. These events generally occur on a small scale, unlike the widespread occurrences seen in U.S. cattle.
What caused the recent jump to cows from another species? Was it a natural, random event as in the past, or were other factors involved?
Gained the Ability to Spread via Aerosols
The original avian H5N1 viruses were not easily transmissible between mammals.
About a decade ago, two virologists, Yoshihiro Kawaoka from the University of Wisconsin in Madison and Ron Fouchier of Erasmus Medical Center in the Netherlands, alarmed the world by conducting high-risk gain-of-function studies on H5N1.
The process was complex. For example, a mutant H5N1 virus was created carrying the specific gene mutation PB2 E627K. It was then passed through ferrets 10 times. After gaining a total of five mutations, the mutant H5N1 virus gained the ability to be transmitted via aerosols or respiratory droplets.
These mutations had only been found in nature, but never all within the same strain. Moreover, their lab manipulation and enhanced ability to transmit via aerosol has resulted in pandemic potential.
In 2011, Paul Keim, a microbial geneticist who chaired the U.S. National Science Advisory Board for Biosecurity (NSABB), expressed concern after reviewing their publications. “I can’t think of another pathogenic organism that is as scary as this one,” he told Science. Having worked on anthrax for many years, he added, “I don’t think that anthrax is scary at all compared to this.”
Publishing these key mutations enables others to replicate the work in their own labs and marks the beginning of the unsettling H5N1 narrative.
The H5N1 clade 2.3.4.4b was first detected in 2013.
Further Manipulation in a Chinese Lab
On April 1, 2021, a three-party project was initiated between the United States, the UK, and China that included the USDA, the U.S. National Poultry Research Center, the Southeast Poultry Research Laboratory (SEPRL) in Georgia, the Chinese Academy of Sciences (CAS), and the Roslin Institute in the UK.
The USDA is sponsoring a grant of $1 million for this project. The SEPRL and Roslin Institute provide expertise in avian immunology genomics and viral transcriptomics analysis.
The actual experiments are conducted in China’s CAS lab. There might be a specific reason for choosing this location.
The project, as we’ll explain later, is also a gain-of-function (GOF) study.
GOF studies on the bird flu virus have triggered broad criticism by the U.S. scientific community since 2011. Richard Ebright, a molecular biologist and laboratory director at the Waksman Institute of Microbiology, also told Science, “This work should never have been done.” From a biosafety perspective, scientists have expressed concern that a new virus generated through research could escape from the lab or that bioterrorists could leverage the published results into a bioweapon for malignant purposes.
In the United States, gain-of-function experiments involving influenza, Middle East respiratory syndrome coronavirus, and severe acute respiratory syndrome coronavirus were banned from October 2014 through December 2017. The moratorium was lifted by the National Institutes of Health (NIH) on Dec. 19, 2017.
Chinese labs often have sufficient technical capacity but face a major challenge due to relatively loose biosecurity regulations.
Former CDC director, Dr. Robert Redfield, recently stated, “Bird flu, I think, is going to be the cause of a great pandemic—where they are teaching these viruses how to be more infectious for humans.”
A Severe, Rapidly Spreading Virus
Chinese scientists are not opposed to doing risky gain-of-function studies on bird flu viruses.
For example, in a study published in Science in May 2013, scientists led by Chen Hualan at Harbin Veterinary Research Institute in Harbin, China, combined the highly lethal but not easily transmissible H5N1 virus with the highly contagious H1N1 swine flu strain, which infected millions of people in 2009.
At least three aspects of the three-party collaborative project study design strongly indicate its gain-of-function nature. However, these may be difficult to discern without reading between the lines.
One significant issue is the experimental approach known as “serial passage.” The process of serial passage research is widely acknowledged by scientists as a tool for gain-of-function studies.
Serial passage involves growing and reproducing the virus from one cell to another or from one animal to another. These studies have high risks of generating mutations that can lead to greater transmissibility, pathogenicity, and zoonotic transmission. The more potent mutants can be selected for the next passage.
As written in their proposal, CAS scientists are responsible for measuring “fitness,” which indicates the outcome of a viral infection—whether it develops faster or slower and whether it results in a severe or mild illness. Samples are collected before and after each round of passages to identify patterns of transmission and pathogenicity. This increases the likelihood of creating mutant H5N1 strains that can cause more severe diseases with faster transmission.
The second clue is linked to the animal models they carefully selected to reproduce the virus—mallard ducks, Chinese geese, and Japanese quail.
The mallard duck is the most abundant migratory and wide-ranging duck on Earth and can crossbreed with 63 other species. It is an asymptomatic carrier harboring many bird flu viruses, potentially allowing more mutated viruses to recombine.
Flu viruses are large, single-stranded RNA viruses comprising an eight-segmented genome. This unique feature of the virus genome implies that it is easy to reassort to one another, resulting in different combinations of genomes, especially when given a perfect condition of many different types of viruses residing in one host.
Furthermore, the Japanese quail has a dual expression of two bird flu virus receptors on both avian and mammalian species. It is such an ideal host that after a series of passage trials, people can identify those strains that are more adaptive to mammalian receptors but not bird receptors.
Therefore, this study design favors the selection of a mutated H5N1 virus that has enhanced tropism for mammalian hosts with a higher pathogenicity or transmissibility.
This is a technologically well-designed study setting to achieve the gain-of-function purpose, in which the study objective appears to be about enhanced surveillance, monitoring, fitness, and vaccine studies.
In addition, this study plans to use live viruses to challenge mallard ducks with low-pathogenic bird flu viruses first, followed by a high-pathogenic virus.
Because the bird flu virus is highly prone to recombination, a genome reassortment among high- and low-pathogenic bird flu viruses could generate new recombinant influenza viruses with unpredictable host tropism or pathogenicity.
Therefore, this creates an even higher potential of generating new gain-of-function mutants.
Since 2021, the bird flu virus H5N1 of clade 2.3.4.4b has had an explosive geographic expansion among wild birds and domestic poultry across Asia, Europe, and Africa, and spread to America at the end of 2021.
Response to Criticism
There has been longstanding criticism of gain-of-function research. Several members of the U.S. Congress have also expressed serious concern about collaborating with the Chinese on bird flu research.
“We are disturbed by recent reports about the U.S. Department of Agriculture’s (USDA) collaboration with the Chinese Communist Party (CCP)-linked Chinese Academy of Sciences (CAS) on bird flu research,” they wrote in an April 12 letter.
“This research, funded by American taxpayers, could potentially generate dangerous new lab-created virus strains that threaten our national security and public health,” they added.
When interviewed by the Science journal in February, the lead investigator denied that they planned to do gain-of-function studies. However, the experimental approach includes “in vivo passage of viruses through mallard ducks and Chinese goose species to predict evolution in natural hosts.”
The lead scientist at CAS involved in this study, Wenjun Liu, emphasized that the Chinese government has strict regulations for lab safety. However, this argument is far from convincing since even a biosafety level 4 lab—the highest safety level—can have serious safety compliance issues, as demonstrated with the Wuhan Institute of Virology and COVID-19.
The recent suspension of funding for scientist Peter Daszak, president of EcoHealth Alliance, sends a clear signal that people distrust virological studies linked to Chinese government-controlled labs.
Increased Pathogenicity
The pathogenicity of H5N1 in animals has increased.
In a 2023 study published in Cell, researchers at the University of Pittsburgh and the Vaccine Research Center of the NIH, used their existing model of cynomolgus macaques to test the effectiveness of the H5N1 vaccine.
In this study, an inhaled aerosol dose of 5.1 log10 plaque-forming units (PFU) caused a strong fever and acute respiratory disease in four out of six macaques, resulting in their deaths. PFU is a method of measuring the amount of the virus.
In comparison, in studies conducted from 2001 to 2014 with cynomolgus macaques, when these monkeys were given high doses of H5N1 (6.5–7.8 log10 PFU) through various routes (nose, throat, mouth, and eyes), they usually developed mild illness, and only 2 out of 49 monkeys died from the infection, based on previous reports.
Compared to the studies done 10 to 23 years ago, a much lower dose used in the 2023 study caused a much higher percentage (half) of the monkeys’ deaths. This indicates that the pathogenicity of the H5N1 virus has dramatically increased.
History Repeated?
While we retrospectively reviewed the timing of the GOF studies in 2012 and 2021 and the outbreaks of H5N1 bird flu viruses in birds and mammals in 2013 and 2021, it is clear that there is a close temporal relationship between them.
This research on the bird flu viruses and current outbreaks in birds and cows should also remind us of the fiercely debated origin of SARS-CoV-2.
A widely discussed, evidence-based viewpoint on the origin of the SARS-CoV-2 virus suggests that bat-derived coronaviruses, previously harmless to humans, gained the ability to infect humans through lab manipulation.
It’s particularly important to consider the current focus of scientific research after experiencing an unprecedented, challenging period due to COVID-19. Some Chinese government-controlled labs are still creating more dangerous viruses and enabling them to spread on a large scale in the name of pandemic preparedness. This raises the question of whether they are truly helping people or creating more diseases.
These alarming facts and circumstances should prompt immediate, thorough investigations into Chinese labs and their potential connection to the H5N1 bird flu outbreak.
In the pursuit of advancing science and researching more effective ways to protect people, such as developing vaccines, the underlying driving force behind such endeavors is often technological competition. However, scientists may have created more problems than solutions for humanity.
In many health articles from The Epoch Times, we emphasize that the best way to prevent a pandemic or viral infection is to focus on improving our health. This includes maintaining a healthy lifestyle, enhancing our natural immunity, and preserving our natural healing abilities.
Editing the virus to enhance its transmissibility and pathogenicity, and researching its pandemic potential, only fuels more fear, rather than resolving the issue.
Ironically, some modern technology can have an extensive negative impact on society. The ability of scientists to conduct GOF research does not justify its necessity.
It’s time for people to wake up.
More About Bird Flu Viruses
There are four types of flu viruses: A, B, C, and D. Based on our current knowledge, only type A can cause global pandemics. A pandemic can occur when an influenza virus has the ability to create long-lasting human-to-human transmission in a population with limited immunity against the virus. In history, three type A flu viruses have triggered human pandemics: H1N1 (1918), H2N2 (1957), and H3N2 (1968).
Influenza A viruses are classified into dozens of subtypes according to two types of glycoproteins on the surface of the virus.
The first glycoprotein, hemagglutinin (H), allows the virus to bind to a celluar surface receptor known as sialic acid and enter the cell. Its name comes from its ability to cause red blood cells to clump together into masses. The second one, neuraminidase (N), is a receptor-destroying protein and enzyme that cleaves the glycosidic bonds of the neuraminic acid, which helps release new viral particles from infected cells. The balance between the H and N function has potential implications for transmission, host adaptation, and pathogenicity between species.
A total of 19 H proteins (H1–H19) and 11 N proteins (N1–N11) have been identified. Different combinations of H and N can be used to name a flu virus. H5N1 has a type 5 H and a type 1 N, so its name is H5N1.
The “H5Nx” nomenclature indicates different neuraminidase types (such as N1, N2, N6, N8) are paired with the H5 protein.
A “clade” is like a branch on a family tree. In a virus family, a clade refers to a group of viruses from a common ancestor with similar characteristics. Clade 2.3.4.4b includes various viruses from H5N1, H5N2, H5N5, H5N6, and H5N8.
Five subtypes of avian influenza A viruses, H5, H6, H7, H9, and H10 are known to have caused human infections.
Bird flu viruses are classified as either low or highly pathogenic avian influenza based on the disease severity they trigger.
The H5 and H7 subtypes are highly pathogenic. Specifically, A(H5N1) and A(H7N9) viruses have caused most of the avian influenza A viral infections reported in people.
HPAI A(H5N6) and LPAI A(H9N2) viruses have also caused human infections in recent years.
Views expressed in this article are the opinions of the author and do not necessarily reflect the views of The Epoch Times or ZeroHedge.